
Transforming treatment for people living with advanced immunological conditions
Windward Bio Expands Immunology Pipeline With WIN027, a Long-Acting, Clinical-Stage Bispecific Targeting TSLP and IL-13
About Windward Bio
Windward Bio is a clinical-stage biotechnology company with deep discovery, development, and commercialization expertise committed to transforming the treatment of people living with advanced immunological conditions. Its lead program is WIN378, a potential best-in-disease, long-acting anti-TSLP monoclonal antibody currently in a Phase 2 trial for asthma. The pipeline also includes WIN027, a clinical-stage, long-acting anti-TSLPxIL-13 bispecific, which has broad therapeutic application across immunological diseases. The company is building a pipeline of long-acting bispecific antibodies, targeting validated biology in respiratory and dermatological conditions. Windward Bio launched in January 2025 with a $200M Series A led by top-tier investors.

Pipeline
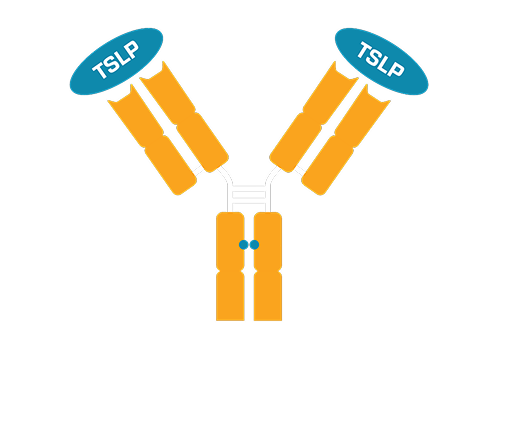
WIN378 is an anti-TSLP antibody with potential best-in-disease dosing frequency
WIN378 is a novel, recombinant, fully human monoclonal antibody designed to advance the standard of care in immunological diseases such as asthma and COPD. By targeting TSLP—a validated driver of inflammation across diverse patient populations—WIN378 builds on the proven therapeutic value of TSLP inhibition.
WIN378 has been engineered to achieve an HLE (half-life extension) and a silenced Fc effector function and is subcutaneously administered. Inhibition of the TSLP ligand has demonstrated clinical benefit in asthma and COPD across diverse inflammatory phenotypes.
WIN378 is currently being evaluated in POLARIS, a global Phase 2 trial in asthma patients.
Fc: fragment crystallizable
Meet the Team
Current Investors









News
Press Release
December 21, 2025
Press Release
July 23, 2025
Press Release
January 10, 2025
For general inquiries, please contact: media@windwardbio.com






